Forms, Policies & Procedures
 Here you will find a repository of forms, policies and procedures related to research at the University of Delaware. This repository draws on sources throughout campus to provide quick and easy access to these resources in a variety of formats, such as html, MSWord and Adobe PDF. We encourage you to explore and use the tools provided to narrow your search by word, resource type or category in order to learn more about the content that governs research at UD.
Here you will find a repository of forms, policies and procedures related to research at the University of Delaware. This repository draws on sources throughout campus to provide quick and easy access to these resources in a variety of formats, such as html, MSWord and Adobe PDF. We encourage you to explore and use the tools provided to narrow your search by word, resource type or category in order to learn more about the content that governs research at UD.
*NOTE: As of October 2020 Google Chrome changed how it handles file downloads. If you encounter difficulties, right click on the “Download” button/link and select “save link as.” Once selected the file download will be executed and can be saved to the desktop. A second method is to use a different browser.
Animal Subjects in Research
For Forms, Policies and Procedures pertaining to Animal Subjects in Research and other resources
Compliance
Conflict of Interest
Contracts and Grant Management
Effort Certification
Export Regulations (ITAR/EAR/OFAC)
Human Subjects in Research
Intellectual Property
Internal Funding
Material Transfer
Reporting Misconduct
Research Administration
Research Agreement Templates
Safety
Students
Templates
University
Procedure: U.S. Department of State
The International Traffic in Arms Regulations (ITAR)
The International Traffic in Arms Regulations (ITAR)
The Department of State is responsible for the export and temporary import of defense articles and services governed by 22 U.S.C. 2778 of the Arms Export Control Act (“AECA”; see the AECA Web page) and Executive Order 13637. The International Traffic in Arms Regulations (“ITAR,” 22 CFR 120-130) implements the AECA. The ITAR is available from the Government Printing Office (GPO) as an annual hardcopy or e-document publication as part of the Code of Federal Regulations (CFR) and as an updated e-document.
Procedure Details:
OWNER: Department of State
RESPONSIBLE OFFICE: Research Office
Procedure Source
Email
Procedure: Research Office
Transportation and Shipment of Biological Materials
Transportation and Shipment of Biological Materials
The goal of the University of Delaware is to ensure the safe transportation of biological materials while complying with all applicable regulations. These shipments may be regulated by the Department of Transportation (DOT), the International Air Transport Association (IATA), and the Centers for Disease Control and Prevention (CDC). International shipments may also necessitate importation or exportation requirements such as permits for the shipments. Below are resources to perform these shipments.
- University of Delaware Guideline for Shipping Biological Materials
- University of Delaware Guideline for Transporting Biological Materials
RESOURCES:
- Centers for Disease Control and Prevention
- Federal Express (FedEx) – Dangerous Goods Section
- International Air Transport Association (IATA) – Dangerous Goods Program
- U.S. Department of Agriculture (USDA) – Animal and Plant Inspection Service
Questions regarding shipment or transportation issues may be addressed to Krista Murray or call 831-1433
Procedure Details:
OWNER: Environmental Health & Safety
RESPONSIBLE OFFICE: Environmental Health & Safety
Procedure Source
Email
Procedure: Research Office
UD Service Maintenance Contract Procedure Advance Payment on Sponsored Projects Internal Guidance
UD Service Maintenance Contract Procedure Advance Payment on Sponsored Projects Internal Guidance
Service/maintenance contracts on sponsored projects are agreements with a vendor to perform services to ensure the equipment’s satisfactory operation during the life of a sponsored project. There are two types of payment options for service/maintenance contracts: advance payment or a payment schedule. This guidance addresses advance payments for equipment contracts.
Advance payment can be advantageous if the vendor offers a significant discount for early payment. The vendor may also require advance payment. Advance payment requires documentation to support why this payment option is required and/or necessary.
If it is determined that advance payment is the optimal course, it must be reasonable, allowable, allocable, and applicable to the sponsored project. To confirm this, the following questions should be considered:
1) Was the associated piece of equipment charged to the project?
2) Is the service/maintenance contract important, necessary, critical, or vital to the
continued operation of the equipment?
3) What are the implications to the sponsored project if the service/maintenance
contract is forfeited?
To be in compliance with Uniform Guidance – Subpart D, E, & F, this documentation should be included in the UD Exchange (UDX) requisition request. A document may be attached, or a comment can be included on the requisition (for example):
“This contract is vital to the project due to [INSERT EXPLANATION]. Research
would halt with any delay due to equipment malfunction because [INSERT
EQUIPMENT NAME] is necessary and integral to project to [INSERT EXPLANATION].”
If the vendor requires advance payment, and there were no other qualified vendor options, please add a note to UDX regarding that. After determining the service / maintenance contract is essential to the project, appropriate allocation of the cost can be managed according to the term of the contract:
1) Contracts covering twelve months or less; or
2) Multi-year contracts covering more than twelve months.
Contracts covering twelve months or less
Service/maintenance contracts that cover twelve months or less can be charged directly to the sponsored project within the same budget period as paid.
Multi-year service/maintenance contracts covering more than twelve months
Multi-year service/maintenance contracts on sponsored projects are allowable. In the event of a multi-year contract, it is recommended, if possible, to seek quarterly or annual payment.
1) If a multi-year contract is advantageous to the project, the justification (cost, service level, etc.) must be provided in UDX for review and approval by the Research Office. The period approved will be allowable to the sponsored project as paid.
2) For multi-year contracts which are disallowed to be charged to the project under (1) above, the first-year costs may be allocated to the sponsored project as paid, while subsequent years must be allocated to a departmental non-sponsored source of funding. Subsequent year costs may be reallocated to the allocable sponsored project within each budget period corresponding to the service/maintenance contract as paid.
Cost allocation to the sponsor code will be as paid.
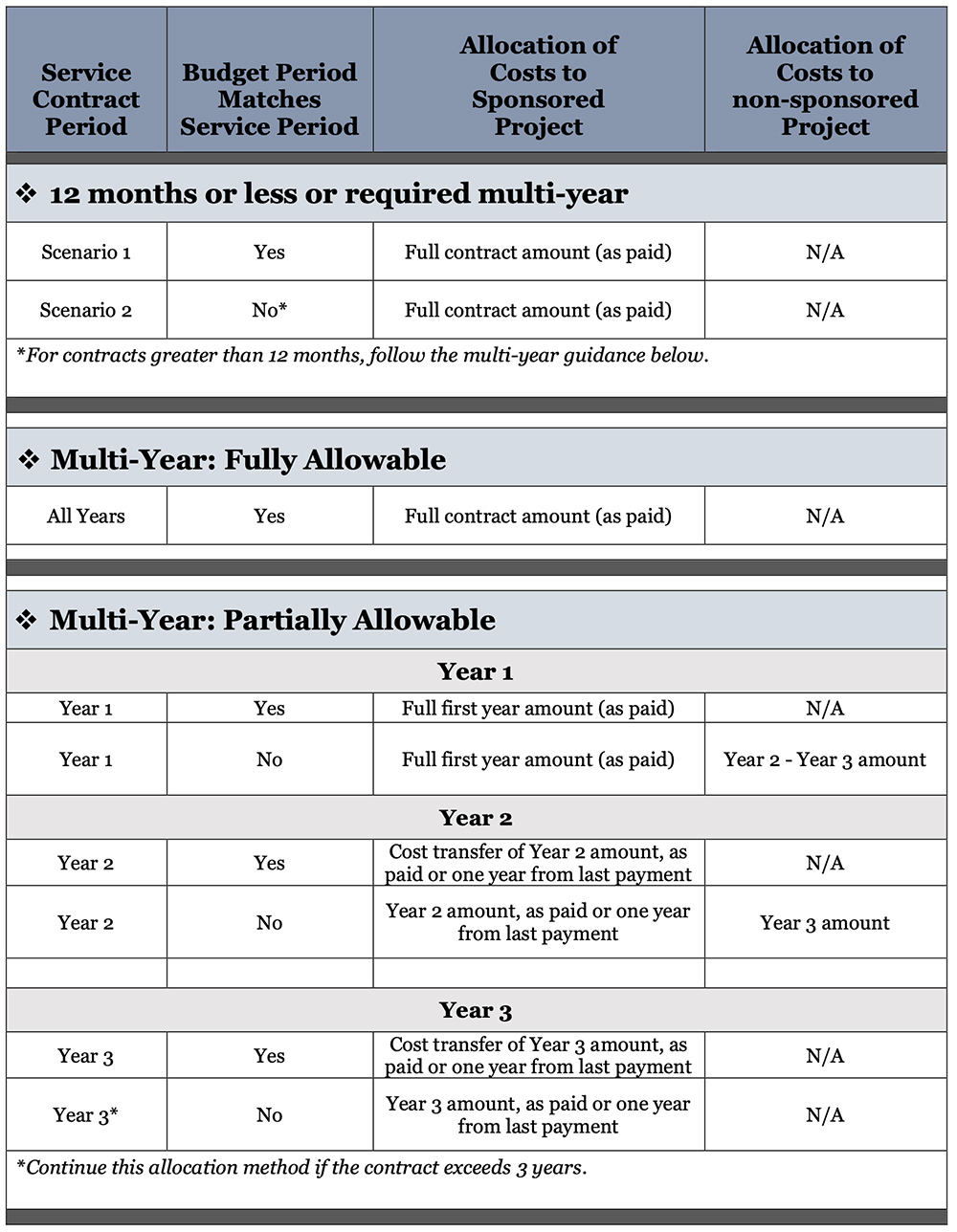
Example Scenarios
Example 1 – Twelve-month contract or less
Invoice received: 03/12/2023
Federal Award: 9021086936
Award period: 03/01/2021-08/31/2025
Maintenance contract period: 04/01/2023-03/31/2024
Annual Maintenance Total: $18,500.00
Accounting: $18,500.00 to award in March 2023 (when invoice received)
Sticking with the main example above, except changing the what ifs below:
What if: invoice received 06/01/2023 and contract period was 06/01/2023-05/31/2024.
Accounting: charge $18,500.00 to award in June 2023
What if: invoice received 06/01/2023 and contract period was 06/25/2023-05/24/2024.
Accounting: charge $18,500.00 to award in June 2023
What if: invoice received 10/01/2024 and contract period was 10/01/2024-09/30/2025.
Accounting: charge 11/12ths of $18,500 to award in October 2024 (award ends 08/31/2025)
Example 2 – Multi-year contract
Invoice received: 05/12/2023
Federal Award: 5021086639
Award period: 07/01/2022-06/30/2026
Maintenance contract period:
Year 1: 04/17/2023- 04/16/2024; $75,250.25
Year 2: 04/17/2024- 04/16/2025; $79,440.48
Year 3: 04/17/2025- 04/16/2026; $80,996.75
Annual Maintenance Total: $235,687.48
Example 2: Accounting- if allowed in full:
Charge full contract amount, $235,687.48, to award in May 2023 (when invoice received) (Allowed in full: justification provided for vital criteria/implications to sponsored project)
Example 2: Accounting- if disallowed in full:
Charge Year 1, $75,250.25 to award in May 2023 (when invoice received)
Charge Years 2-3 total, $160,437.23 to a department discretionary code in May 2023 (when invoice received)
Note: with multi-year contracts, the out years charge must be allocated to a department discretionary purpose code and reallocated to the award yearly corresponding to the service contract year making sure it falls within the award period.
Procedure Details:
OWNER: Research Office
RESPONSIBLE OFFICE: Research Administration
ORIGINATION DATE: July 21, 2023
Procedure Source Email
Procedure: NIH
Updated Requirements for NIH “Other Support”
Updated Requirements for NIH “Other Support”
BACKGROUND AND IMPORTANCE
The National Institutes of Health (NIH) needs to understand the degree to which the Principal Investigator (PI) or other senior/key personnel have support and/or resources from other sources for their research activities. The primary drivers cited for seeking this information are to ensure proper commitment of time (avoiding both under-commitment and over-commitment) by the senior personnel working on the project and avoiding duplication of funding for research requests. Given the recent additional scrutiny by the federal government on foreign influence (and particularly, scans for an inappropriate foreign influence), additional information is being requested. These requirements continue to evolve, and this page will be updated to reflect the latest information.
DEFINITION of “Other Support”:
(NIH Grants Policy Statement, December 2021)
“…includes all resources made available to researcher or senior key personnel in support of and/or related to all of their research endeavors, regardless of whether or not they have monetary value and regardless of whether they are based at the institution the researcher identifies for the current grant. Other support does not include training awards, prizes, start-up support from the US based institution, or gifts (note: Gifts are resources provided where there is no expectation of anything (e.g. time, services, specific research activities, money, etc.) in return).”
Data that is requested for each reportable activity includes (in this order):
|
|
Active AND Pending |
*** Consultants (Research Only) |
*** Resources/ Financial Support |
In-Kind Active and Pending |
|
Title |
|
|
|
|
|
Major Goals |
|
|
|
|
|
Status of Support |
|
|
|
|
|
Project number |
|
|
|
|
|
Name of Principal Investigator (contact PI if multi-PI study) |
|
|
|
|
|
Source of support (fund source) |
|
|
|
|
|
Primary Place of Performance |
|
|
|
|
|
Proposal Start and End Date |
|
|
|
Provide when applicable |
|
Total Award Amount (see below) *New |
|
|
|
Estimated value of in-kind contribution |
|
Person Months devoted by reporting investigator per budget period (see NIH guidance) **New |
|
|
|
(or reasonable estimate) |
|
Entity (Foreign or Domestic) |
|
|
|
|
|
Description |
|
|
|
|
*For each reportable activity, include the total award amount (including Facilities and Administrative Costs) for the entire award period.
**Number of person-months per budget period to be devoted (e.g., 1.5 months).
***State “None” if Consultants and/or Resources do not apply.
Also required are:
*Overlap: After listing all support, summarize any potential overlap with the active or pending projects and activities, other positions, affiliations, and resources and this application in terms of the science, budget, or an individual’s committed effort.
*Signature: Each PD/PI or other senior/key personnel must electronically sign their respective Other Support form prior to submission. This signature certifies that the statements are true, complete and accurate.
WHO MUST REPORT:
- Principal Investigator
- All other senior/key personnel listed in a grant application except Other Significant Contributors and Program Directors, training faculty, and other individuals involved in the oversight of training grants
- All senior/key personnel, excluding consultants, in progress reports when there has been a change in active support except Program Directors, training faculty, and other individuals involved in the oversight of training grants
WHEN TO REPORT:
Expected starting May 25, 2021 and required starting January 25, 2022:
- Just-in-Time (upon request by NIH after proposal submission but prior to award).
- After Just-in-Time but prior to receipt of award (reporting required only if changes are substantive* in nature)
- Via Prior Approval Request for substantive* changes that occur during the award period but prior to the due date of the next RPPR.
- Research Performance Progress Reports (annual progress reports) – report changes only
- Upon request by NIH
*While NIH does not define “substantive”, the concept is that the change is of a magnitude that NIH might prudently need to review the new arrangements to ascertain whether the existence, timing or the amount of NIH’s award might need to change in light of the new information (e.g. substantive new support that alters the reporting investigators availability or where a prudent person might question whether there is scientific or financial overlap)
FORMS/INSTRUCTIONS FOR REPORTING
NIH Other Support Format Page:
https://grants.nih.gov/grants/forms/othersupport.htm
WHAT TO REPORT:
Other Support includes all resources made available to a researcher in support of and/or related to all of their research endeavors, regardless of whether or not they have monetary value and regardless of whether they are based at the institution the researcher identifies for the current grant. This includes but is not limited to:
- Resources and/or financial support from all foreign and domestic entities, that are available to the researcher. This includes but is not limited to, financial support for laboratory personnel, and provision of high-value materials that are not freely available (e.g., biologics, chemical, model systems, technology, etc.). Institutional resources, such as core facilities or shared equipment that are made broadly available, should not be included in Other Support, but rather listed under Facilities and Other Resources.
- Consulting agreements, when the PD/PI or other senior/key personnel will be conducting research as part of the consulting activities. Non-research consulting activities are not Other Support.
- In-kind contributions, e.g. office/laboratory space, equipment, supplies, or employees or students supported by an outside source. If the time commitment or dollar value of the in-kind contribution is not readily ascertainable, the recipient must provide reasonable estimates.
- If in-kind contributions are intended for use on the project being proposed to NIH in this application, the information must be included as part of the Facilities and Other Resources or Equipment section of the application and need not be replicated on this form.
- In-kind contributions not intended for use on the project/proposal being proposed in this application must be reported below. If the time commitment or dollar value is not readily ascertainable, reasonable estimates should be provided.
Other support does not include training awards, prizes, or gifts. Gifts are resources provided where there is no expectation of anything (e.g. time, services, specific research activities, money, etc.) in return. An item or service given with the expectation of an associated time commitment is not a gift and is instead an in-kind contribution and must be reported as such.
SUPPORTING DOCUMENTATION:
Provide copies of contracts specific to senior/key-personnel foreign appointments and/or employment with a foreign institution for all foreign activities and resources that are reported in Other Support. If they are not in English, recipients must provide translated copies. This does not include personal service contracts, or employment contracts for fellows supported by foreign entities. Supporting Documentation should be provided as a PDF following the Other Support form. This documentation must be supplied to the Grants Analyst from the senior/key-person at the time other support is due. If you have questions regarding foreign components (appointments or other employment), please contact Research Office Regulatory Affairs.
ADDITONAL GUIDANCE FROM NIH:
NIH has a webpage devoted to Other Support, which includes FAQs and instructions, as well as a page that includes examples on what to disclose.
See also:
- NOT-OD-21-073: Upcoming Changes to the Biographical Sketch and Other Support Format Page for Due Dates on or after May 25, 2021
- University of Delaware Research Office, Foreign Involvement
- NOT-OD-19-114: Reminders of NIH Policies on Other Support and on Policies related to Financial Conflicts of Interest and Foreign Components.
- Mike Lauer’s 7/19 Blog post entitled, “Clarifying Long-Standing NIH Policies on Disclosing Other Support”
- NIH Protecting Biomedical Innovation (including helpful reporting at-a-glance chart)
IN SUMMARY:
| Other Support Changes |
Effective |
Effective |
|
Disclose all sources of Other Support and related information, including outside activities (e.g. consulting, visiting professorships) if conducting research, In-Kind support, and gifts if donor expects anything in return (e.g. time, services, research). |
Required |
Required |
|
Follow new format |
Not required |
Required |
|
Separate sections for Project/Proposal Support and In-Kind |
Not required |
Required |
|
Completed Support for the last 3 years |
Not required |
Required |
|
Submit copies of agreements for investigator’s foreign appointment/employment if listed as Other Support |
Not required |
Required |
|
If agreement not in English, provide translated copy |
Not required |
Required |
|
Redact confidential information expect for key provisions (e.g. award amount, dates) |
Not required |
Required |
|
When requested, provide copies of agreements for investigator’s foreign appointment/employment if listed as Other Support |
Required |
Required |
|
Each senior/key person electronically signs PDF of their Other Support |
Not required |
Required |
|
If Other Support signed, flatten PDF prior to submission |
Required |
Required |
|
Submit updated Other Support immediately upon learning a source of Other Support for an active NIH grant was not disclosed |
Required |
Required |
Procedure Details:
OWNER: National Institutes of Health
RESPONSIBLE OFFICE: Research Office
ORIGINATION DATE: May 24, 2021
REVISION DATE(S): 1/10/2022, 1/19/2022
Procedure Source
Email
Procedure: U.S. Department of Commerce
US Bureau of Industry and Security – Export Administration Regulations (EAR)
US Bureau of Industry and Security – Export Administration Regulations (EAR)
These are the unofficial electronic EAR files created by BIS. The legally official text of the EAR is provided via the Federal Register publications. Incorporation of revisions pursuant to Federal Register regulatory publications are completed by BIS within 72 hours to the best of our abilities. While we strive for perfection, we do make mistakes from time to time. You may email any errors that you find.
Procedure Details:
OWNER: US Bureau of Industry and Security
RESPONSIBLE OFFICE: Research Office
Procedure Source
Email

